Why Equi Sciences?
We are focused on ensuring that diversity is well represented when it comes to patients enrolled on clinical studies.
Bridging the Minority Gap in Clinical Research & Drug Development
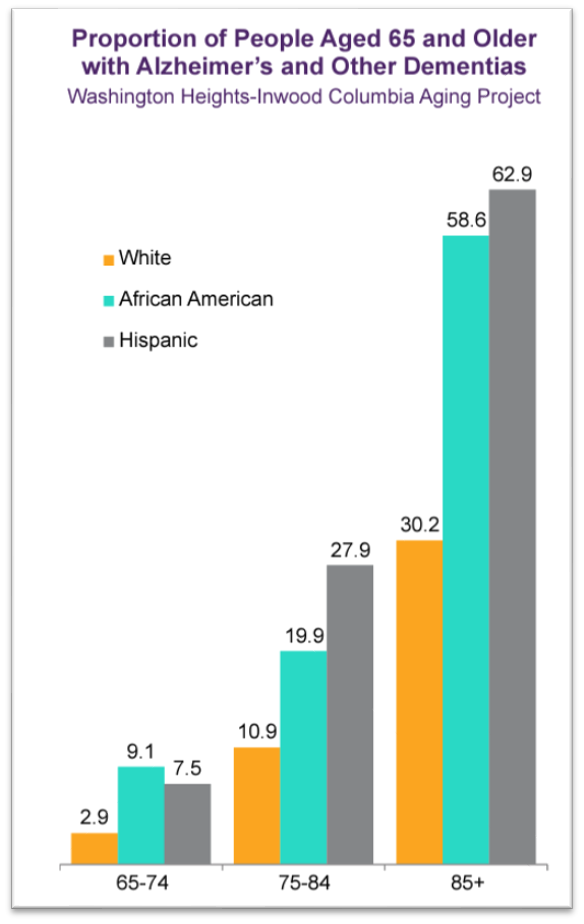
Equi Sciences CRO is dedicated to bridging the minority gap in clinical research and drug development. We believe that diversity in clinical trials is crucial for ensuring the safety, efficacy, and generalizability of medical treatments. It matters because genetic variability among populations can affect treatment outcomes. At Equi Sciences, we are committed to promoting diversity in clinical trials and contributing to the advancement of medical research.
Diversity in Clinical Trials
Three key reasons why diversity matters in clinical trials
- Genetic variability among populations can affect how individuals respond to medications. Genetic markers that influence drug metabolism, efficacy, and the risk of adverse reactions may be more prevalent in specific ethnic or racial groups. Without diverse representation in clinical trials, treatments might be less effective or have unanticipated side effects in underrepresented groups.
- Studies have shown that clinical trials that include participants of varying ages, races, ethnicities, genders, and with different comorbidities produce results that are more generalizable. This ensures that healthcare providers can confidently prescribe treatments, knowing they have been tested across diverse groups.
- Promoting diversity in clinical trials is also an ethical imperative because all individuals should have the opportunity to participate in research that could benefit their health and the health of others who share their characteristics.

Compliance With New FDA Regulations





Equi Sciences is revolutionizing the world of clinical research and drug development. We are dedicated to pushing boundaries and driving innovation by embracing diversity. Recognizing the importance of inclusivity in clinical trials, we are committed to ensuring the safety, efficacy, and relevancy of medical treatments.
Subscribe to Our Newsletter
Subscribe to our newsletter for the latest updates and exclusive offers.